Iron Oxide Nanoparticles Synthesis – (Fe3O4) by using Iron Chloride and Ammonia via Coprecipitation Method
Cite This in Your Publication
Iron Oxide Nanoparticles Synthesis – (Fe3O4) by using Iron Chloride and Ammonia via Coprecipitation Method - InstaNANO. https://instanano.com/all/nanomaterial-synthesis/metal-oxide/iron-oxide-nanoparticles-1/ (accessed May 3rd, 2024).
Iron Oxide Nanoparticles Synthesis – (Fe3O4) by using Iron Chloride and Ammonia via Coprecipitation Method - InstaNANO. https://instanano.com/all/nanomaterial-synthesis/metal-oxide/iron-oxide-nanoparticles-1/ (accessed May 3rd, 2024).
Iron Oxide (Fe3O4) Nanoparticles Synthesis by using Iron Chloride as precursor and Ammonia as reducing agent via Coprecipitation Method.
-
CHECK LISTIron(II) chloride (FeCl2.4H20), Iron(III) chloride (FeCl3.6H20), 25% Ammonium Hydroxide Solution (NH4OH), Nitrogen (N2) gas, Deionized Water, RB Flask, Magnetic Stirrer with Temperature option, Centrifuge, Furnace.
-
STEP 1.Take 0.5g Iron(II) chloride and 1.35g Iron(III) chloride in 50ml of deionized Water at 60°C under inert atmosphere (N2 or Ar gas flow).
-
STEP 2.After 30 minutes of proper mixing, Inject 33ml of Ammonium Hydroxide Solution (Ammonium Hydroxide Solution is 25% diluted which is commonly available).
-
STEP 3.After 2 hours of proper mixing at maximum rpm, color of solution turned into Black.
-
STEP 4.Filter out the Black color powder, wash it with deionized Water and Ethanol several times to remove the unwanted impurities.
-
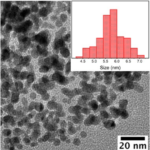
RESULTSBlack color powder indicated the formation of Fe3O4 Nanoparticles. Average size during the synthesis was found to be 10-15 nanometers.
-
Factors Affecting SynthesisPresence of Inert Gas: Presence of inert gas highly effect the synthesis of Nanoparticles as it can change the amount of Oxygen during the synthesis. Without using the inert gas proper particles formation and crystallization does not occur.
Concentration of Ammonia Solution: Higher concentration of Ammonia solution leads to larger particles size. If you get higher particle then you can reduce the amount of ammonia solution. -
NOTE: All the experiments should be done under the guidance of lab Incharge; and proper lab safety instructions.