Zinc Oxide Nanoparticles Synthesis – ZnO by using Zinc Nitrate and Potassium hydroxide via Chemical Reduction Method
Cite This in Your Publication
Zinc Oxide Nanoparticles Synthesis – ZnO by using Zinc Nitrate and Potassium hydroxide via Chemical Reduction Method - InstaNANO. https://instanano.com/all/nanomaterial-synthesis/metal-oxide/zinc-oxide-nanoparticles-2/ (accessed February 26th, 2026).
Zinc Oxide Nanoparticles Synthesis – ZnO by using Zinc Nitrate and Potassium hydroxide via Chemical Reduction Method - InstaNANO. https://instanano.com/all/nanomaterial-synthesis/metal-oxide/zinc-oxide-nanoparticles-2/ (accessed February 26th, 2026).
Zinc Oxide (ZnO) Nanoparticles Synthesis by using Zinc Nitrate as precursor and Potassium hydroxide as reducing agent via Chemical Reduction Method.
- CHECK LISTZinc Nitrate (Zn(NO3)2.6H2O), Potassium hydroxide, Deionized Water, RB Flask, Magnetic stirrer
- STEP 1.Make 0.2M Zinc Nitrate solution at room temperature.
- STEP 2.Make 0.4M Potassium hydroxide solution in another RB Flask.
- STEP 3.Add Potassium hydroxide solution (prepared in step-2) into Zinc Nitrate solution (prepared in step-1). Stop adding KOH, when white suspension is formed.
- STEP 4.This white suspension are pre-formed ZnO Nanomaprticles, which can be seperated out at 5000-7000rpm in centrifuge
- STEP 5.This white powder obtained after centrifuge is calcined at 500°C for 3 hours in air.
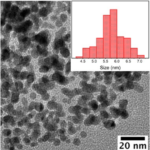
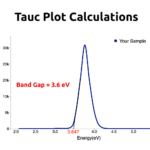
- RESULTSSpherical 30nm size Zinc Oxide (ZnO) Nanoparticles are formed during this process. UV-Vis absorption of ZnO Nanoparticles are around 340nm.
- NOTE: All the experiments should be done under the guidance of lab Incharge; and proper lab safety instructions.



















Please recommend morphological based nanoparticles like sphere, rod, tubes.