Nickel Nanoparticles Green Synthesis by using Nickel Chloride and Desmodium Gangeticum Leaves via Biological Method
Cite This in Your Publication
Nickel Nanoparticles Green Synthesis by using Nickel Chloride and Desmodium Gangeticum Leaves via Biological Method - InstaNANO. https://instanano.com/all/nanomaterial-synthesis/metal/nickel-nanoparticles-1/ (accessed April 29th, 2024).
Nickel Nanoparticles Green Synthesis by using Nickel Chloride and Desmodium Gangeticum Leaves via Biological Method - InstaNANO. https://instanano.com/all/nanomaterial-synthesis/metal/nickel-nanoparticles-1/ (accessed April 29th, 2024).
Nickel Nanoparticles Green Synthesis by using Nickel Chloride as precursor and Desmodium Gangeticum Leaves extract as reducing and stabilising agent via Biological Method.
-
CHECK LISTFresh leaves of Desmodium Gangeticum (Linn), Nickel Chloride (anhydrous), Deionized Water, RB Flask, Dropper, Magnetic Stirrer with Temperature option, Filter Paper
-
STEP 1.Take fresh leaves of Desmodium Gangeticum. Wash properly and cut them into small pieces.
-
STEP 2.Take 25g leaves pieces in 100ml of deionized water and boil the leaves around 80-90ºC for 5-10 minutes.
-
STEP 3.When the water turns GREEN, filter it out and use it as reducing and capping agent for Nanoparticles synthesis.
-
STEP 4.Take 0.5g Nickel Chloride in 100ml deionized water; and set the temperature 80ºC.
-
STEP 5.Add leaf extract drop wise slowly (prepared in step 3) into Nickel Chloride solution, until the color changes to light GRAY.
-
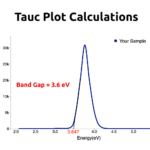
RESULTSThe Gray color indicated the formation of Nickel Nanoparticles in water. UV-Vis spectroscopy absorption peak would be obtained around 270nm.
-
Factors Affecting SynthesisSpecies of Plant/Tree: Different types of Plants/Trees are having different types of chemical composition and concentration of specific chemical in its leaves. So different species of Plants/Trees can give the different particle size, size distribution. Even morphology can also be changed by using leaf extract of different plants/trees.
Concentration of Nickel Chloride: Nickel Chloride is used as the precursor in this synthesis. Higher the concentration of Nickel Chloride, more the chances of agglomeration; further leads to bulky size particles.
Concentration of Leaves in Water: Concentration of leaves in water is very important for Nanoparticles synthesis. Higher concentration of leaves in water, leads to higher concentration of reducing and capping agent in water.
Temperature: The temperature is another important factor in the synthesis of Nanoparticles, change in temperature leads to change in the particles size; also higher temperature leads to faster chemical Kinetics. -
NOTE: All the experiments should be done under the guidance of lab Incharge; and proper lab safety instructions.